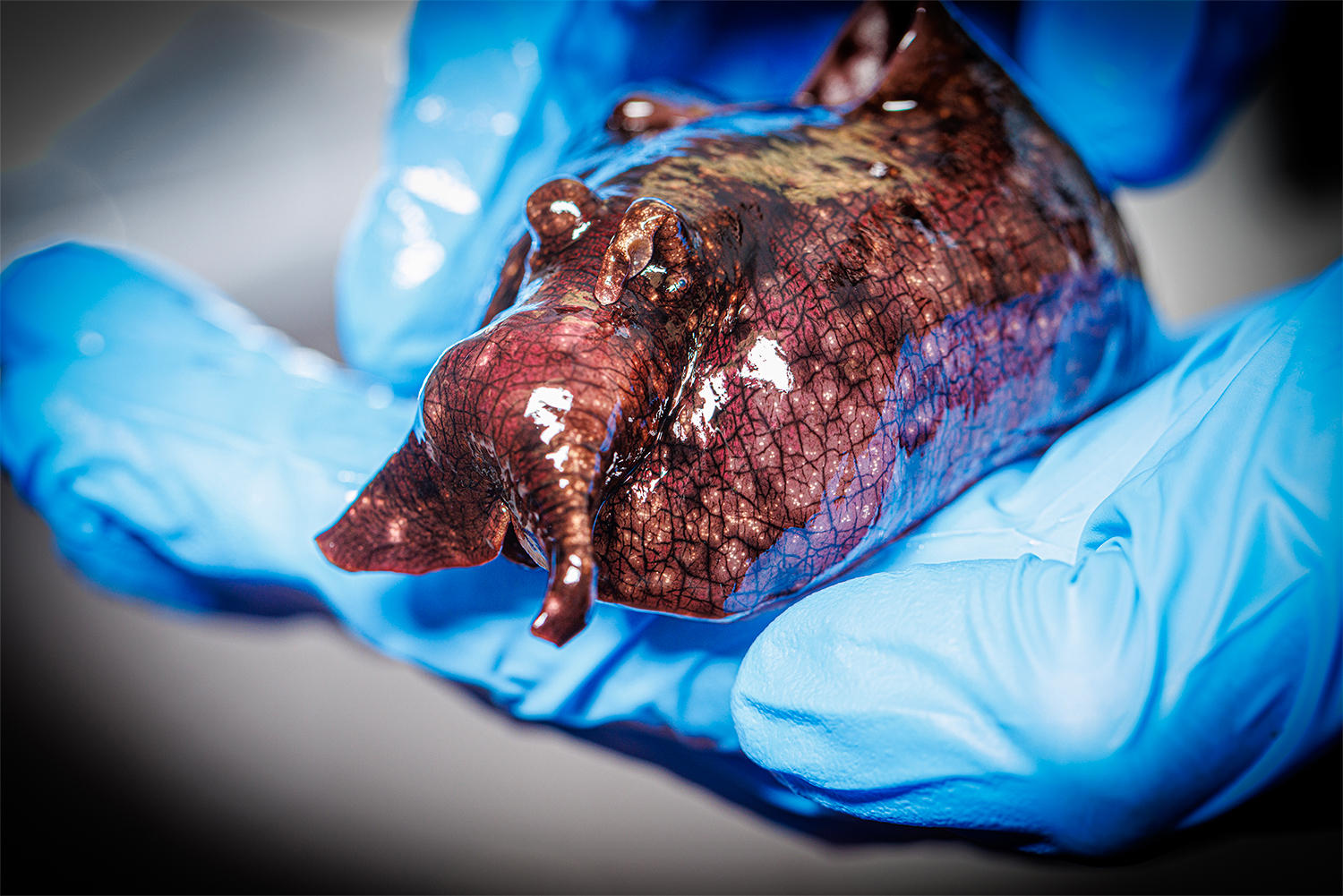
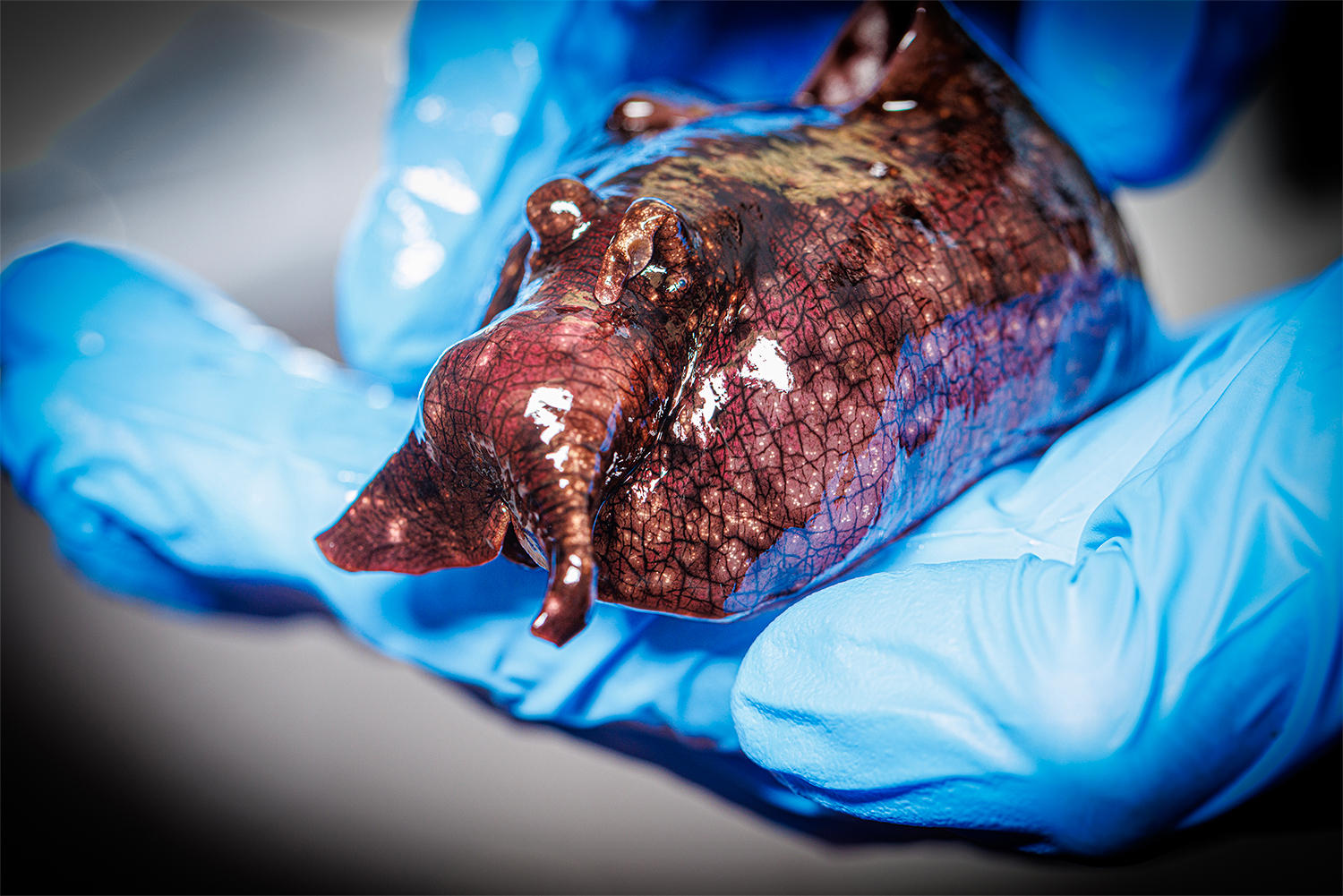
Nebraska 화학자들은 분자에 대한 매우 자연스럽고 약간의 변화가 신경 전달 물질이 활성화할 신경 세포 수용체를 지시할 수 있음을 발견했습니다. 연구팀은 화학자 James Chico가 보관하고 있는 바다 민달팽이 종에서 이 현상을 발견했지만, 이 발견은 다양한 동물, 심지어 인간에게도 적용되어야 합니다. 크레딧: 크레이그 챈들러 | 네브래스카 대학교 – 링컨
신경 신호는 거울 이미지 분자에 의해 변경될 수 있습니다.
일부 바다 민달팽이의 도움으로 네브래스카-링컨 대학의 화학자들은 생체 분자에 가능한 가장 작은 변화 중 하나가 상상할 수 있는 가장 큰 결과 중 하나인 뉴런의 활성화를 유도할 수 있다는 것을 발견했습니다.
그들의 연구는 짧은 사슬인 펩타이드에 초점을 맞췄습니다.[{” attribute=””>amino acids capable of transmitting signals between cells, including neurons. These peptides are present in the central nervous systems and bloodstreams of most animals. An amino acid in a peptide can take on one of two forms, L or D, which consist of the same atoms connected in the same way but arranged in mirror-image orientations.
Chemists often think of those two orientations as the left and right hands of a molecule. The L orientation is by far the more common in peptides, to the point of being considered the default. But when enzymes do flip an L to a D, the seemingly minor about-face can turn, say, a potentially therapeutic molecule into a toxic one, or vice versa.

A basic rendering of the left and right hands of the same amino acid. Credit: University of Nebraska-Lincoln
Now, Husker chemists James Checco, Baba Yussif, and Cole Blasing have revealed a whole new role for that molecular mirroring. For the first time, the team has shown that the orientation of a single amino acid — in this case, one of dozens found in the neuropeptide of a sea slug — can dictate the likelihood that the peptide activates one neuron receptor versus another. Because different types of receptors are responsible for different neuronal activities, the finding points to another means by which a brain or nervous system can regulate the labyrinthine, life-sustaining communication among its cells.
“We’ve discovered a new way that biology works,” said Checco, assistant professor of chemistry at Nebraska. “It’s nature’s way of helping to make sure that the peptide goes to one signaling pathway versus the other. And understanding more about that biology will help us to be able to take advantage of it for future applications.”
Checco’s interest in neuropeptide signaling dates back to his time as a postdoctoral researcher, when he came across the first study to show evidence of a peptide with a D-amino acid activating a neuron receptor in sea slugs. That particular receptor responded to the peptide only when it contained the D-amino acid, making its flip from L to D akin to an on/off switch.

Nebraska chemists James Checco (center), Baba Yussif (right) and Cole Blasing. Credit: Craig Chandler | University of Nebraska–Lincoln
Eventually, Checco himself would identify a second such receptor. Unlike the one that had initially sparked his interest, Checco’s receptor responded both to a peptide featuring all L-amino acids and the same peptide with a single D. But the receptor was also more responsive to the all-L peptide, activating when introduced to smaller concentrations of it than its D-containing counterpart. Instead of an on/off switch, Checco seemed to have found something closer to a dimmer.
“We were left wondering: Is this the whole story?” Checco said. “What’s really going on? Why make this D molecule if it’s even worse at activating the receptor?”
The team’s newest findings, detailed in the journal Proceedings of the National Academy of Sciences, hint at an answer inspired by a hypothesis. Maybe, the team thought, there were other receptors in the sea slug sensitive to that D-containing peptide. If so, maybe some of those receptors would respond differently to it.
Yussif, a doctoral candidate in chemistry, went to work searching for sea slug receptors whose genetic blueprints resembled those of the one Checco had uncovered. He ultimately narrowed down a list of candidates, which the team then cloned and managed to express in cells before introducing them to the same D-containing peptide as before. One of the receptors responded. But this receptor — in an almost mirror-image performance of Checco’s original — responded much more favorably to the D-containing peptide than its all-L peer.
“You can see a pretty dramatic shift,” Checco said, “where now the D is, in fact, much more potent than the L at activating this new receptor.”
In effect, the team realized, the orientation of that lone amino acid was directing its peptide to activate either one receptor or the other. In its all-L state, the neurotransmitter preferred Checco’s original. When that certain L turned D, on the other hand, it went for Yussif’s new candidate instead.
Central nervous systems rely on different types of neurotransmitters to send various signals to various receptors, with dopamine and serotonin among those best-known in humans. Given the radical complexity and delicacy of the signaling in many animals, though, Checco said it makes some sense that they might evolve equally sophisticated ways of fine-tuning the signals sent by even a single neuropeptide.
“These sorts of communication processes need to be very, very highly regulated,” Checco said. “You need to make the right molecule. It needs to be released at the right time. It needs to be released at the right site. It needs to degrade, actually, in a certain amount of time, so that you don’t have too much signaling.
“So you have all this regulation,” he said, “and now this is a whole new level of it.”
Unfortunately for Checco and others like him, naturally occurring peptides that contain D-amino acids are difficult to identify using the instrumentation readily available to most labs. He suspects it’s one reason that, at least to date, no D-containing peptides have been found in people. He also suspects that will change — and that, when it does, it could help researchers better grasp both the function and disease-related dysfunction of signaling in the brain.
“I think it is likely that we will find peptides with this kind of modification in humans,” Checco said. “And that’s going to potentially open up new therapeutic avenues in terms of that specific target. Understanding more about how these things are functioning could be exciting there.”
In the meantime, Checco, Yussif, and Blasing, a senior double-majoring in biochemistry and chemistry, are busy trying to answer other questions. For starters, they wonder whether an all-L versus D-containing peptide — even those equally likely to activate a receptor — might activate that receptor in different ways, with different cellular consequences. And the search for receptors won’t stop, either.
“This is one receptor system, but there are others,” Checco said. “So I think we want to start to extend and discover new receptors for more of these peptides, to really get a bigger picture about how this modification influences signaling and function.
“Where I really want to go long-term with this project,” he said, “is to get a better idea, across all of biology, of what this modification does.”
Reference: “Endogenous l- to d-amino acid residue isomerization modulates selectivity between distinct neuropeptide receptor family members” by Baba M. Yussif, Cole V. Blasing and James W. Checco, 6 March 2023, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2217604120

“요은 베이컨과 알코올에 대한 전문 지식을 가진 닌자입니다. 그의 탐험적인 성격은 다양한 경험을 통해 대중 문화에 대한 깊은 애정과 지식을 얻게 해주었습니다. 그는 자랑스러운 탐험가로서, 새로운 문화와 경험을 적극적으로 탐구하며, 대중 문화에 대한 그의 열정은 그의 작품 속에서도 느낄 수 있습니다.”
/cloudfront-us-east-2.images.arcpublishing.com/reuters/VHEUP7RJDROHNMEIYKARPKJOKI.jpg)


![궤도를 도는 태양광 모듈은 태양의 섬세한 코로나를 놀랍도록 자세하게 포착합니다. [Video] 궤도를 도는 태양광 모듈은 태양의 섬세한 코로나를 놀랍도록 자세하게 포착합니다. [Video]](https://scitechdaily.com/images/ESA-Solar-Orbiter-scaled.jpg)




